
About Kayla Fioravanti:
Kayla Fioravanti is the co-founder of Ology Essentials and is an award-winning author, certified aromatherapist and cosmetic formulator. She is the author of The Art, Science and Business of Aromatherapy and the co-author of the Amazon #1 New Release, The Unspoken Truth About Essential Oils. To learn more about Kayla, visit her website at: https://ift.tt/3md8tPp
The Truth About Hand Sanitizers
In March 2020, the pandemic called coronavirus (COVID-19) triggered a rush on hand sanitizers. Consumers and businesses alike rapidly jumped on the DIY hand sanitizer bandwagon. Unsafe and inaccurate DIY recipes swept social media. The U.S. Food & Drug Administration (FDA) warned consumers, “FDA recommends that consumers do not make their own hand sanitizer. If made incorrectly, hand sanitizer can be ineffective, and there have been reports of skin burns from homemade hand sanitizer. The agency lacks verifiable information on the methods being used to prepare hand sanitizer at home and whether they are safe for use on human skin”1
Antimicrobial Sanitizers as OTC Drugs
In the United States, the Center for Drug Evaluation and Research (CDER), a division of the U.S. Food and Drug Administration (FDA), regulates all antimicrobial sanitizers as over-the-counter drugs (OTC). Any and all topical products making anti-microbial claims must follow all of the regulations. The FDA also regulates what type of claims can be made on OTC hand sanitizers. In fact, PURELL® received a strong warning letter from the FDA for breaking those rules. On their product web pages for PURELL® Healthcare Advanced Hand Sanitizer the company claimed it: “Kills more than 99.99% of most common germs that may cause illness in a healthcare setting, including MRSA & VRE.” There were also more unsubstantiated claims that converted their OTC product into an unapproved new drug.2
During the pandemic, businesses also started making hand sanitizers without complying with the Center for Drug Evaluation and Research (CDER) regulations of over-the-counter (OTC) drugs. The FDA began sending out warning letters to businesses who were selling Unapproved and Misbranded Products Related to Coronavirus Disease 2019 (COVID-19). Between March 6, 2020 and June 30, 2020 a total of forty-three warning letters went out to companies requiring immediate cessation of all claims that misbranded products in violation of the FD&C Act on social media, website, and in all printed material.3
The Federal Trade Commission (FTC) also has regulatory power over the claims made about products. As of June 30, 2020, there were sixty-five companies who were cited by the FTC for breaking the FTC Act. According to the FTC, “It is unlawful under the FTC Act . . . to advertise that a product can prevent, treat, or cure human disease unless you possess competent and reliable scientific evidence, including, when appropriate, well-controlled human clinical studies, substantiating that the claims are true at the time they are made. For COVID-19, no such study is currently known to exist for the product identified above. Thus, any coronavirus-related prevention or treatment claims regarding such product is not supported by competent and reliable scientific evidence. You must immediately cease making all such claims.”5
As small business owners it is vital that we follow the laws that govern products and that we share formulation advice that is completely accurate and safe. The unsound formulation advice and selling of unproven DIY hand sanitizers puts liability on your business for products that may or may not mitigate a disease, let alone coronavirus (COVID-19).
Temporary Guidance on Preparation and Distribution of Hand Sanitizer
In response to the hand sanitizer shortage, the U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) issued a Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry in March of 2020. This measure gave an avenue for businesses that were not previously regulated by the FDA as drug manufacturers to have temporary guidance on the preparation and distribution of hand sanitizer.
According to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry, action will not be taken by the FDA for the duration of the COVID-19 public health emergency against companies that produce hand sanitizer with very specific conditions set by the FDA. I will break down the basics here, but I have also included the full text.
The Key Points of the Temporary Guidance for Hand Sanitizers for Businesses
The simplified key points that anyone considering selling hand sanitizer must meet include:
- Only a specific list of ingredients is allowed.
- The alcohol must be denatured and meet the standards set for in the temporary guidance.
- Only formulas that are consistent with the World Health Organization’s (WHO) recommendations are allowed.
- Absolutely no active or inactive ingredients may be used (including essential oils).
- There must be accurate record keeping of all batches.
- Testing using accurate methods of analysis must be run on every batch to ensure the correct level of alcohol.
- All manufacturing must be done under sanitary conditions with appropriate equipment.
- The final formula must be aqueous and not be a gel, foam, or aerosol spray.
- Labeling must use the standards for the principal display and drug panels set forth in Appendix A, B, C, or D which can be currently found on page 16 of the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry
- The facility and product must be registered in the FDA Drug Registration and Listing System.7
Updates to the Temporary Guidelines
On June 1, 2020 the FDA did an update on the document to further clarify several details including what kind of alcohols are safe for use for hand sanitizers. This was very likely triggered by the warning letter against the company Eskbiochem due to the potential presence of a toxic substance known as methanol (wood alcohol) that is toxic both through skin absorption and ingestion.8 In the updated information, the FDA allowed for alcohol (ethanol) produced for consumption and alcohol derived from synthetic processes that met United States Pharmacopeia (USP) and Food Chemicals Codex (FCC) grade. In addition, they made a path for alcohol produced by facilities that normally produce fuel or technical grade alcohol (ethanol) as long it is produced by fermentation or distillated used for consumables, it contains no other additives or chemicals, it meets USP or FCC grade requirements, and the alcohol has been screened for impurities.
Types of Alcohol
The form of alcohol used is critical to the success of a hand sanitizer formula. If you are making hand sanitizer for your personal use it is important to be aware that rubbing alcohol, a.k.a. isopropyl alcohol, and the alcohol that you drink are very different. The alcohol that you drink is ethyl alcohol (C2H5OH) and rubbing alcohol is isopropyl alcohol (C3H8O). Another alcohol that you may see on the market is denatured alcohol. It has been denatured to deter people from drinking it. The Poison Control website warns that isopropyl alcohol is poisonous in small amounts to children and also poisonous for adults.
“Please, if you are stockpiling alcohol for hand sanitizers, be extra cautious that children do not have access to isopropyl alcohol.” - Kayla Fioravanti
Alcohol Content by Numbers
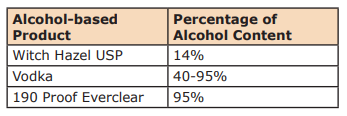
When you look at alcohol you can find the percentage of alcohol in consumable liquor by checking the proof on the label. A label that reads 50% alcohol by volume is 100-proof. Why is this important? One, because you must reach at least the minimum level of alcohol for the hand sanitizer to be effective. If your alcohol is too weak the math will never work out. And two, you need to know how much alcohol is in a finished product, but also the fact that water and essential oils do not mix. This is just a fact of nature. So many DIY hand sanitizers’ recipes on the internet are recommending the use of essential oils. There are so many things wrong with these recipes. One, dangerous levels of essential oils are being recommended, and two, the essential oils are not being dispersed or emulsified into the finished product. Essential oils left improperly diluted and/or dispersed increase your risk of injury or sensitization.
Don’t be discouraged by the standards set forth in the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry. It is very doable. My company was able to register a hand sanitizer with significant ease compared to any other over-the-counter product in the past. And if you really want to provide hand sanitizers it is possible to work with a registered company to sell it either wholesale or private label.
Selling Bulk Hand Sanitizer by a Registered Company
So, what about repackaging bulk hand sanitizer manufacturer by a registered company? I have received countless questions on the legality of repackaging bulk hand sanitizers. I turned to other experts to confirm my suspicious. The consensus was that anything repackaged would be out of compliance with the National Drug Code (NDC). The NDC is a universal product identifier for human drugs in the United States. It uses a three-segment number that identifies the labeler (manufacturer, re-packager, or distributor), the product code (identifying strength, dosage, form, and formulation of drug for a company), and the commercial package size.9 If you choose to sell the bulk hand sanitizer in the packaging you purchase it in, or if you have the registered company private label it for you, that would then meet the National Drug Code standards.
Essential Oils and COVID_19
As an aromatherapist, I simply must touch on the use of essential oils to prevent or cure coronavirus (COVID-19). The FDA is not allowing the use of any essential  oils or other additives in the temporary measure to any hand sanitizer. If you previously were a registered drug manufacturer of OTC hand sanitizer and that formula uses essential oils, then you can continue manufacturing and selling that product. If you are new and using the temporary measures, then you cannot add any material to fragrance or enhance the formula.
oils or other additives in the temporary measure to any hand sanitizer. If you previously were a registered drug manufacturer of OTC hand sanitizer and that formula uses essential oils, then you can continue manufacturing and selling that product. If you are new and using the temporary measures, then you cannot add any material to fragrance or enhance the formula.
Also, keep in mind that absolutely no essential oils have been clinically proven to destroy the coronavirus (COVID-19). Shannon Becker, PhD RA further explains, “Essential oils considered to be ‘antiviral’ are not universal virus killers. Before we explain the existing research on ‘antiviral essential oils,’ it is important to clarify the difference between virucidal and antiviral. ‘Antiviral’ means that a compound inhibits the proliferation of a virus, while ‘virucidal’ means a virus is destroyed or deactivated. In many instances, essential oils may be effective in killing one specific virus, but not another. Tea tree (Melaleuca alternifolia) essential oil inhibits the proliferation of influenza viruses inside cells (making it antiviral), but only modestly inhibits HSV-1 and HSV-2 (Garozzo et al 2009). Tea tree essential oil was not able to inhibit proliferation of the non-enveloped viruses’ poliovirus 1, adenovirus 2, echovirus 9, and Coxsackie B1 (Garozzo et al 2009).”10
Business Insurance for Temporary Hand Sanitizer Production and Sales
From one business owner to another, I would be remiss to skip advising you to check if your business insurance will allow for you to sell this temporary OTC formula. I do know that Indie Business Network (IBN) members with product liability insurance through IBN’s program with Veracity Insurance Services have coverage for handmade hand sanitizer, so long as they are in compliance with FDA’s Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency. Check with your insurance manufacturing hand sanitizer. Remember that this is a temporary measure. Do not totally revamp your business to solely depend on hand sanitizer sales long term. This is a temporary way to pivot during the crisis caused by the COVID 19 pandemic. Also, don’t make any outrageous claims. Just keep it simple.
QUOTED DIRECTLY FROM THE FDA Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry sourced from https://ift.tt/2BuK3OR
1. The hand sanitizer is manufactured using only the following ingredients in the preparation of the product
a. Select one of two options:
(i) Alcohol (ethanol) that is not less than 94.9% ethanol by volume11; OR
(ii) United States Pharmacopeia (USP grade) Isopropyl Alcohol (IPA)12,13
b. Glycerin (glycerol) USP or Food Chemical Codex (FCC) (also known as “food grade”)
c. Hydrogen peroxide.
d. Sterile water (e.g., by boiling, distillation, or other process that results in water that meets the specifications for Purified Water USP). Water should be used as quickly as possible after it is rendered sterile or purified.
Additional Considerations for Ingredients in Preparation of the Product:
Alcohol (ethanol) that is produced using fermentation and distillation processes typically used for consumable goods, and that is made in a facility used for producing consumable goods, may be considered for use in hand sanitizer.
Alcohol derived from synthetic processes may be considered for use in hand sanitizer only if it meets USP or FCC grade.
Alcohol produced in facilities normally producing fuel or technical grade alcohol (ethanol) may be considered for use in hand sanitizer provided the following circumstances are present:
(i) the alcohol is produced using fermentation and distillation processes typically used for consumable goods, and no other additives or other chemicals have been added to the ethanol;
(ii) the alcohol meets USP or FCC17 grade requirements or the conditions in
Attachment 1; and,
(iii) the alcohol has been screened for any other potentially harmful impurities not
specified in the USP or FCC requirements but potentially present based on the
specific manufacturing environment.
Ingredients that are described as only meeting American Chemical Society (ACS) grade
standards should generally not be used in hand sanitizers.
2. The alcohol (ethanol) is denatured either by the alcohol producer or at the point of production of the finished hand sanitizer product. Alcohol and Tobacco Tax and Trade
Bureau regulations in 27 CFR part 20 and 21, respectively, describe requirements pertaining to, and provide a number of formulas for, denaturing alcohol. Formulas for use in hand sanitizers under FDA’s temporary policies include:
a. Formula No. 40A or No. 40B with or without the tert-butyl alcohol
b. Formula No. 3C (isopropyl alcohol)
Denaturing is critical because there have been reports of adverse events, including deaths, from ingestion of hand sanitizer. Most reports are from unintentional ingestion by young children. The alcohol should be denatured at either (1) the point of production by the alcohol production firm or (2) the point of manufacture or compounding of the hand sanitizer. Attachment 2 provides more information on the formulas used to denature alcohol before it is used in alcohol-based hand sanitizers. Attachment 2 reproduces Appendix C from FDA guidance for industry Temporary Policy for Manufacture of Alcohol for Incorporation into Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19).
3. The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations.
a. Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (80%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20; or Isopropyl Alcohol (75%, v/v) in an aqueous solution.9
b. Glycerol (1.45% v/v).10
c. Hydrogen peroxide (0.125% v/v).
d. Sterile distilled water or boiled cold water.
4. The firm does not add other active or inactive ingredients, such as ingredients to improve the smell or taste, due to the risk of accidental ingestion in children. Different or additional ingredients may impact the quality and potency of the product.
5. The firm pays particular attention to ensure the ethanol or isopropyl alcohol active ingredient is correct and the correct amount of the active ingredient is used. A simple record should be used to document key steps and controls to assure each batch matches the formula developed for the drug product.
6. The hand sanitizer is prepared under sanitary conditions and equipment utilized is well maintained and fit for this purpose.
7. The firm uses the most accurate method of analysis available at the site for verification of alcohol content in samples of the finished drug product before each batch is released for distribution. Methods can include gas chromatography (GC), alcoholmeter, hydrometer, or other chemical analysis of at least equivalent accuracy. The sample tested can be performed on in-process material before filling into the final containers to be distributed.
8. The hand sanitizer product is produced as an aqueous solution and not as a gel, foam, or aerosol spray. The firm packages the finished hand sanitizer product in packaging appropriate for liquid drug products that will seal sufficiently to prevent evaporation of the alcohol or IPA. Manual pump sprays that seal sufficiently to prevent evaporation are consistent with this policy.
9. The hand sanitizer is labeled consistent with the attached labeling in Appendix A
(Labeling for Ethanol Formulation Consumer Use), Appendix B (Labeling for Isopropyl
Alcohol Formulation Consumer Use), Appendix C (Labeling for Ethanol Formulation
Health Care Personnel Hand Rub Use), or Appendix D (Labeling for Isopropyl Alcohol Formulation Health Care Personnel Hand Rub Use).
10. Firms register their facility and list these products in the FDA Drug Registration and listing System (DRLS, https://ift.tt/3md8zXh). Firms that are required to register their foreign establishment with FDA must list all known importers in the United States in their registration in accordance with Section 510(i)(1)(A) of the FD&C Act. See also 21 CFR 207.25(h)(2). Upon completion of registration and listing, firms receive automatic confirmation from the FDA and do not need to wait for a further communication from FDA before the firm can begin to distribute these products. FDA relies on registration and listing information to help manage drug shortages, monitor safety issues that may arise with product distributed to the public, and manage product recalls, among other important FDA public safety activities. Our help desk is standing by to assist with facilitating this process and can be contacted by sending an email to: edrls@fda.hhs.gov.6
References
U.S & Drug Administration. “Q&A for Consumers: Hand Sanitizers and COVID-19.” Accessed July 6, 2020 from: https://ift.tt/2Xv89QN
U.S & Drug Administration. “Warning Letter, GOJO Industries Inc, MARCS-CMS 599132—JANUARY 17, 2020.” Accessed July 6, 2020 from: https://ift.tt/33na91b
U.S & Drug Administration. “Warning Letters.” Accessed July 6, 2020 from: https://ift.tt/2JlXDYz
Federal Trade Commission. “FTC Coronavirus Warning Letters to Companies.” Accessed July 6, 2020 from: https://ift.tt/37eZS8j.
Federal Trade Commission. “Thinking about making Coronavirus claims? Read the latest FTC warning letters first.” Accessed July 6, 2020 from: https://ift.tt/2xmDP1X
U.S & Drug Administration. “Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry.” Accessed July 6, 2020 from: https://ift.tt/3q0Tqux
U.S & Drug Administration. “Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry.” Accessed July 6, 2020 from: https://ift.tt/2BuK3OR
U.S & Drug Administration. “FDA advises consumers not to use hand sanitizer products manufactured by Eskbiochem” Accessed July 6, 2020 from: https://ift.tt/3dnbmIa
Drugs. “National Drug Codes Explained.” Accessed July 6, 2020 from: https://ift.tt/36ccRZ6
Becker, Shannon, PhD RA; (2020), Tisserand Institute. “Essential Oils and Coronaviruses.” Accessed July 7, 2020 from: https://ift.tt/2MVgLve
Source link
[ad_2]
source https://earn8online.com/index.php/190508/the-truth-about-hand-sanitizers/
No comments:
Post a Comment